Faster research with integrated patch-clamp workstations
Why do patch clamp?
Next to fluorescence imaging, patch clamp electrophysiology has become THE workhorse technology for cell physiology research. Indeed, even cellular imaging methods often build on patch-clamp experiments. For studying the function of ion channels, neuromodulation, synaptic plasticity and neuronal connectivity, no technique offers a temporal resolution and signal-to-noise that gets close to patch clamp.
As a result, when studying the functional ins and outs of most cells – plant cells, myocytes, neurons, cardiomyocytes – no matter if in intact tissue or isolated, then more likely than not, patch-clamp will be one of the key techniques to consider.
From different recording configurations (i.e. whole-cell, excised patch, cell-attached, loose-seal) to combining recordings with genetic analysis or imaging, the range of techniques is steadily growing.
Where is patch clamp heading?
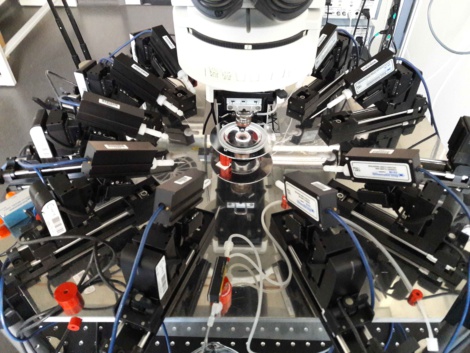
A patch clamp rig for simultaneous recordings from up to ten neurons (Image kindly provided by the lab of Prof. Jörg Geiger, Charité Berlin).
Patch-clamp recordings are well into their “middle-age”, with over forty years of continuously growing use. Nearly three decades ago the first combination of whole-cell recordings with fluorescence imaging was the first time patch-clamp “levelled up”. In parallel, the unofficial competition to record from ever smaller structures was on – first proximal dendrites, distal dendrites, on to tiny basal dendrites, axons and finally synaptic boutons… in culture, in slices, in vivo.
Today, whole-cell recordings are often combined with genetic analysis of the cells after recording, bringing single cell resolution to transcriptomics. At the same time, patch-clamp is increasingly used in studies aimed at acquiring large data sets – be it for screening of drugs or transgenic constructs or unravelling neuronal or other cellular networks.
It is a truism to state that all these methods (electrical recordings, genetic analysis, imaging, and increased throughput) will play an ever growing role in cell physiology and pharmacology.
Issues with patch clamp recordings
Patch-clamp recordings are not always easy. Even when experiments and data collection were a matter of recording from a single, visually identified cell, there are many things that can go wrong. Electrical noise, drifting and unstable manipulators, jerky movement when trying to patch a cell, vibration during movements… all of these things will slow down data acquisition and impact the quality of that data.
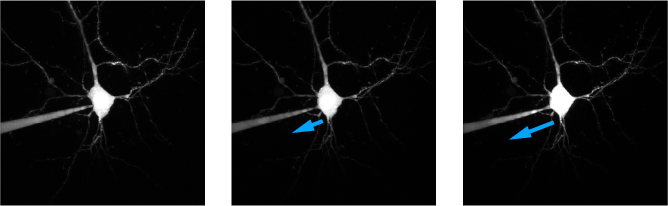
Drifting pipette: A layer 5 neuron patched with a dye-filled pipette. In the first, left, panel the pipette is snugged up against the cell. In the successive images the pipette is drifting away from the cell, stretching the membrane and leading to an increased access resistance(image kindly provided by Dr. Grit Bornschein, Carl Ludwig Institute, Leipzig University).
The impact of such issues caused by different parts of the recording setup not performing well, and not working well together is exasperated by the increasing complexity of experiments as described above. Ultimately, this all compounds to slow down experiments.
At the same time, the higher throughput achieved by recording from multiple cells and locations at the same time, also means repetitive tasks such as changing pipettes, approaching a cell and forming a seal are taking up substantial amounts of time and are becoming a bottle neck for data throughput.
Finally, a problem particularly felt in old institutions in large cities: control electronics to drive all this equipment take up space. As most equipment used in patch-clamp relies on 19″ control racks, a system for recording from eight or ten targets at the same time will often require more space for the control electronics to be housed than for the actual experimental setup.
Integrated patch-clamp workstations are the solution
Tackling these issues is where integrated patch-clamp workstations come into play: a combination of multiple micromanipulators, microscope, and sample stage, all linked by a unifying control system, working together to ensure the complete system runs efficiently and smoothly.
This is taken to the next level if the different devices are based on today’s cutting edge technology: The stability and high repeatability afforded by using closed-loop motion control – both for manipulators and microscopes removes the typical pain points. Even in lower throughput experiments, where one or two cells are recorded from at the same time.

A fully integrated patch clamp workstation for parallel recordings from up to 8 cells.
Here, the use of piezo technology in particular makes it possible to have manipulators that are drift-free for hours and do not generate detectable electrical noise. The stability and smoothness of manipulators is only one aspect – ensuring smooth motion of the sample stage is key. Especially, if cells being recorded from have expansive dendrites or are spread out over large areas. If all of this can be controlled with electronics that take up less space than a small PC tower, even better.
When increasing the throughput, these highly repeatable positioning devices make it possible to easily automate repetitive tasks. Allowing researchers to focus on the important parts of the experiment.
The final step to increased throughput is to automate even more sophisticated parts of experiments: Recent developments have made it possible to reuse patch pipettes rather than manually replacing them – increasing the throughput in connectivity studies by over 75%.
Finally, the automation of seal formation that has been made possible through the use of finely calibrated pressure controllers brings a new level of reproducibility to patch-clamp recordings. That these pressure controllers paired with automated motion sequences allow genetic material to be automatically extracted from cells after the recordings and deposited in PCR vials goes without saying.
If the hardware provides a unified control interface, ideally one that can be easily accessed from third-party or self-written software, the interplay of these advanced technologies allow for automation of complex sequences. This can be achieved through a powerful and easy to use software development kit (SDK) with wrappers for commonly used programming languages, such as Python. Open source software packages implementing this level of control, along with machine vision and data acquisition capabilities already exists for those wanting to make the jump into the next generation of patch clamp experiments.
So where does this take us? Patch experiments will remain a technique that requires skill and experience. Even the most integrated systems won’t turn electrophysiological research into a “push a button and you are done” discipline. But it will remove many of those headaches and allow you to focus on the important thing: doing the science.
Related products
uM-Patch workstations
Fully integrated and configured patch clamp rigs
- Reliable – make experiments reproducible
- Easy to use – get patching sooner
- Class-leading accuracy – easily navigate your slices
uMs-FN1 Microscopes
The industry-leading patch clamp microscopes
- Super smooth – move your sample without losing recordings
- Compact – slim body and no need for bulky control racks
- Intuitive and easy to use – stay focused on your experiment
uMp Manipulators
The World’s most stable patch clamp manipulators
- Zero drift technology – the pipette stays where you put it
- Ultra compact – fit the manipulator where you need it
- Super scalable – add another manipulator when you need it (no new controllers needed)

